What are Redox Reactions and Why is Redox Modulation So Important? (press title)
Redox reactions are chemical oxidation–reduction processes that involve the transfer of electrons. During these reactions, highly reactive molecules called free radicals are generated, including reactive oxygen species (ROS) and reactive nitrogen species (RNS). Within cells, redox reactions keep a dynamic balance between oxidants and reductants. This balance is supported by both endogenous antioxidant molecules and enzymes such as oxidoreductases and dehydrogenases, which work together to ensure that oxidized and reduced products stay in equilibrium, preserving the cellular activity required for survival.
Beyond keeping balance, redox reactions are also essential for cellular communication. Acting as a fundamental regulatory system, redox-active molecules—including ROS and RNS—function as signaling messengers that send information both between and within cells. These signals can start cascades of events that regulate cell growth, division, migration, or even cell death.
Electron transfer reactions also form the core of energy metabolism. During glycolysis, the citric acid cycle (Krebs or TCA cycle), oxidative phosphorylation, and mitochondrial energy production, free radicals are inevitably generated. In addition, redox reactions drive immune responses, regulate metabolism, and take part in programmed cell death. Thus, ROS serve as a double-edged sword: while they are traditionally linked to oxidative stress and cellular damage, at controlled physiological levels they play essential, reversible roles in cell signaling and regulation.
In addition to serving as the foundation for internal antioxidant defense, redox reactions are essential for counteracting external sources of oxidative stress. These sources range from environmental exposures to air pollution, pesticides, noise, acoustic blasts, and radiation (including excessive sunlight) to lifestyle factors such as smoking, alcohol consumption, stress, aging, poor diet, and adverse drug effects. Medical conditions, including physical injury, obesity, diabetes, cardiovascular disease, inflammatory disorders, and autoimmune disease, further contribute to oxidative stress.
All of these factors increase the generation of reactive radicals such as superoxide anion and nitric oxide. Metal ion imbalance (iron, zinc, copper) further adds to the burden by promoting hydroxyl radical formation. When oxidation exceeds antioxidant defenses, cells undergo DNA damage, lipid peroxidation, protein modifications, and multiple forms of cell death, including iron-dependent ferroptosis. These molecular changes lead to tissue injury and disease, manifesting as vision loss (corneal, lens, and retinal damage), hearing impairment, neurological and cognitive decline, chronic inflammation, and cancer development.
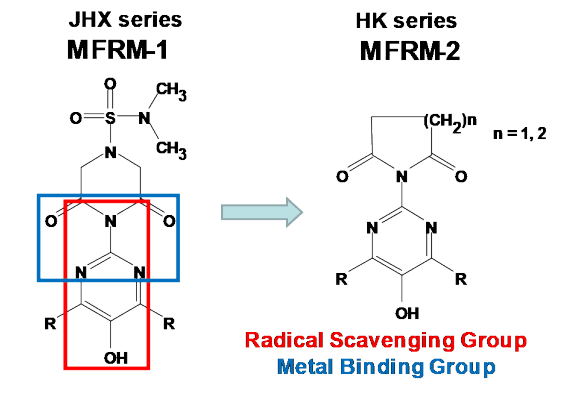
To address redox modulation, Therapeutic Vision has developed a new class of synthetic compounds called Multifunctional Redox Modulators (MFRMs). These MFRMs not only scavenge free radicals but also independently sequester and redistribute the transition metals iron, copper, and zinc. Beyond neutralizing free radicals, MFRMs also counteract neurotoxic zinc complexes found in amyloid beta plaques and protect mitochondrial function against manganese poisoning. These compounds are orally active and reach therapeutic levels in sensory cell tissues, including the eyes, ears, and central nervous system (CNS). Their structures are shown below: